Healthy Volunteers
ERG site CPMI routinely conducts Phase I studies that require overnight stays, sometimes up to 40 days.
120-bed
clinical pharmacology unit
800+
studies with normal
healthy volunteers
5,000+
database of normal
healthy volunteers
Having conducted over 2000 Phase I studies, ERG is known throughout the industry for excelling in these trials. We have access to a wide range of healthy subject profiles, and, whatever your compound, we stand ready to help you explore its clinical pharmacology.
Demonstrated Expertise in Study Designs
- Absolute Bioavailability
- Bioequivalence
- Cardiac Safety, Thorough QT/QTc
- Drug-Drug Interaction
- Elderly Healthy Cohorts in Proof-of-Concept/Proof-of-Mechanism
- ETOH Interaction
- First-to-File
- First-in-Man
- Food Effect
- Patch Irritability
- Pharmacokinetic/Pharmacodynamic
- Relative Bioavailability
- SAD/MAD
- Tasting
Healthy Cohorts in CNS Drug Development
As a neuroscience leader, ERG partners with its clients throughout the CNS drug-development lifecycle, from first-in-man SAD/MAD studies through proof-of-concept/proof-of-mechanism and large Phase III programs; we are also adept at the full suite of clinical pharmacology trials, including drug-drug interaction, food effect, cardiac safety, DDI, TQT, and abuse liability trials.
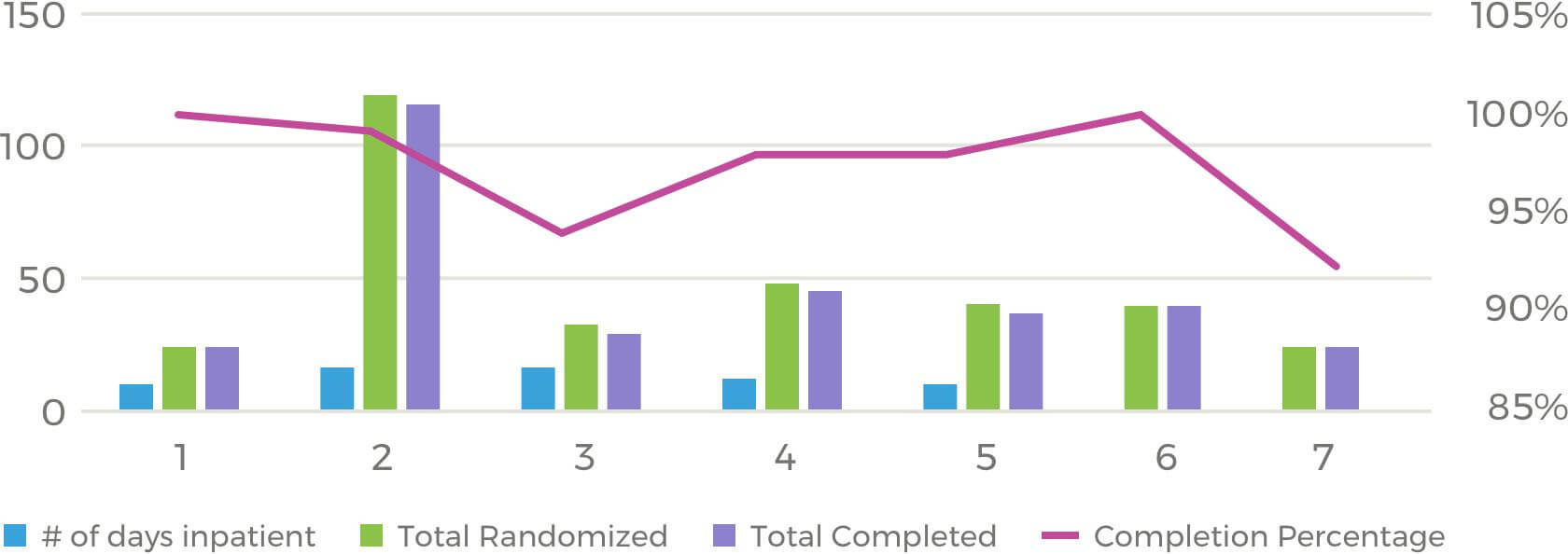
ERG site CPMI hit 97% of their enrollment target for NHV trials within the last year
Targeted Patient Recruitment
With an active database of over 5,000 normal healthy volunteers of all types and profiles, ERG is able to swiftly enroll studies, taking just weeks from contract execution to first patient in (FPI).
Spotlight
Stacey Dilzer
- Sought-after operational leader in early-phase execution and study design for healthy volunteer trials
- Expert in the management and supervision of all phase I-IV clinical trials
- Extensive clinical operations career
Case Study: Bioequivalence Trial
Study compared commercial and development formulations (TDH)
- 4-week enrollment period
- 76 subjects enrolled
- 1216 total inpatient nights
